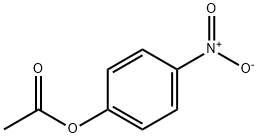
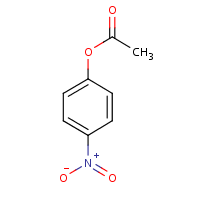
830-03-5 | 4-Nitrophenyl Acetate | NSC 2633; p-Acetoxynitrobenzene; p-Nitrobenzene Acetate; p-Nitrophenol Acetate; p-Nitrophenyl Acetate; p-Nitrophenyl Ester Acetic Acid; p-Nitrophenol Acetate; | C₈H₇NO₄ | TRC

Kinetic Isotope Effects for Acyl Transfer from p-Nitrophenyl Acetate to Hydroxylamine Show a pH-Dependent Change in Mechanism | Journal of the American Chemical Society

Molecules | Free Full-Text | Biosensors and Bioassays Based on Lipases, Principles and Applications, a Review
![PDF] Solvent effects on ester linkage of 4-nitrophenyl acetate in aqueous and ethanol solutions with imidazole and hydroxide ion as nucleophiles | Semantic Scholar PDF] Solvent effects on ester linkage of 4-nitrophenyl acetate in aqueous and ethanol solutions with imidazole and hydroxide ion as nucleophiles | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/6f2da9470bf5ccf676050416195bca719ef55d96/2-Table1-1.png)
PDF] Solvent effects on ester linkage of 4-nitrophenyl acetate in aqueous and ethanol solutions with imidazole and hydroxide ion as nucleophiles | Semantic Scholar

Effect of medium on reactivity for alkaline hydrolysis of p-nitrophenyl acetate and S-p-nitrophenyl thioacetate in DMSO–H2O mixtures of varying compositions: ground state and transition state contributions











![Hydrolysis of p-nitrophenyl acetate [47]. | Download Scientific Diagram Hydrolysis of p-nitrophenyl acetate [47]. | Download Scientific Diagram](https://www.researchgate.net/publication/332187950/figure/fig10/AS:743864423952384@1554362507117/Hydrolysis-of-p-nitrophenyl-acetate-47.png)